Do you rely on end-testing your finished product as the primary quality step? If so, you should consider a change!
There are strong benefits to shifting to a quality method that provides visibility upstream to the entire manufacturing process. Not only are quality issue root causes identified more quickly, but also waste and scrap batches are considerably reduced. Called “Quality by Design (QbD),” the goal is instead to have quality integrated into not only the product, but throughout the manufacturing process.
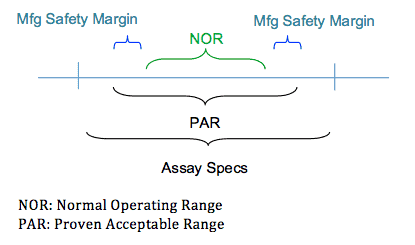
At a very basic level this means your manufacturing capabilities must fall within your assay specifications. This is critical; you do not want any variability in your manufacturing process to lead to assay/product failure. In this graphic, you can see how different parameters bound and define the product assay specifications.
The fundamental takeaway is the better you understand your process and its variables, the fewer manufacturing failures will occur.
The International Conference on Harmonization (ICH) published a scientific approach to product development and manufacturing using the principles of Quality by Design in the document, ICH Q8(R2). The FDA has adopted this as a ‘Guidance Document’.
Due to their impact on patient health and outcomes, Lab Developed Tests (LDTs) and In vitro Diagnostic Tests (IVDs) are increasingly coming under the scrutiny of the FDA. Therefore, more and more companies developing these tests are following the ICH Q8(R2) guidelines. This process not only improves the quality of a product, but also speeds time-to-market. And the same QbD principles are not restricted to regulated products- they strengthen Research Use Only (RUO) products as well!
If you have any questions on how Argonaut can help you navigate or leverage Quality by Design, please contact us!



