Argonaut is a leading CMO in aseptic fill finish services. With a highly experienced team leading the process, we deliver precision filling with minimal line losses, maintaining high product quality and process efficiency. Our facilities are FDA inspected, FDA registered and is ISO 13485 compliant, upholding the highest quality standards. With Annex 1-compliant filling and containment, we prioritize contamination control, risk management, and GMP excellence- ensuring your products meet regulatory expectations and achieve market approval with confidence
Following formulation and filtration in our dedicated segregated suites, Argonaut’s highly experienced team ensures precision filling with minimal line losses. Currently, we offer vial filling, and with the installation and qualification of our additional Annex 1-compliant filling line in Q4 2025, we will expand our capabilities to include prefilled syringes and cartridges. Our team also provides comprehensive post-filling services, including packaging, GMP-compliant storage, logistics, and distribution support.

EU Annex 1 compliant



Our commitment to quality is not just a statement; it’s a way of life at Argonaut. We have implemented a rigorous quality management system that spans every aspect of our organization, from sourcing raw materials to the final product release.

In addition to a team which has decades of experience handling high value products, we have invested in advanced automated aseptic fill finish manufacturing equipment, personnel, and robust quality/regulatory systems. We provide our clients with:

| Fill Finish Services | Vials, syringes, cartridges |
| Capacity | 500-12,600 per hour |
| Enclosures | ISO 5, Grade A Isolators |
| API Loss | Near zero line loss |
| Additional Services | Formulation, packaging, terminal sterilization, cGMP storage, logistics |
| Certifications | FDA inspected, FDA registered, CA FDB license, ISO 13485, EU Annex 1 compliant |

Argonaut’s Bausch+Ströbel VarioSys filling line paired with a SKAN isolator utilizes the highest quality ready-to-use (RTU) vials in a variety of sizes. Using high quality primary packaging components reduces cosmetic defects and glass particulates that can occur in lower quality vials.
Argonaut has carefully optimized tubing lengths and diameters to virtually eliminate line loss and dead volume, resulting in extremely high product yields.
The Bausch+Ströbel VarioSys performs 100% non-destructive in-line weight checks, resulting in maximum yield and extremely consistent fill volumes.
Filled and stoppered vials are crimped in a separate isolator compartment. This spatial segregation from the vial filling process helps avoid potential particulate intrusion due to crimping.
Environmental monitoring (EM) activities are integrated into the line to avoid operators interfacing with multiple pieces of equipment.

Filling station in action
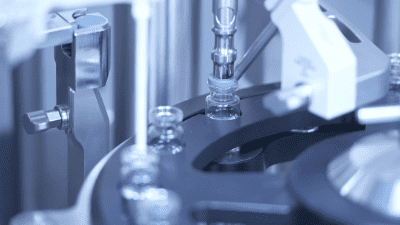
Filling and gas overlay station and stopper placement in action
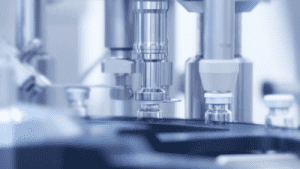
Cap crimping station in action
CDMOs play a crucial role in ensuring the quality of the products they manufacture and maintaining compliance with applicable regulations.
To achieve this, reputable CDMOs like Argonaut implement a comprehensive approach that includes the following elements:
Quality Management System (QMS): A robust QMS is the backbone of a CDMO’s quality and compliance efforts. The QMS includes policies, procedures, and guidelines that govern every aspect of the life science and IVD manufacturing process, from raw materials sourcing to finished product release.
Compliance with Good Manufacturing Practices (GMP): CDMOs adhere to GMP guidelines and other relevant industry standards to ensure that products are consistently manufactured according to quality requirements. GMP guidelines cover various aspects of the production process, including personnel qualifications, equipment maintenance, and record-keeping.
Regulatory expertise: CDMOs employ experienced regulatory affairs professionals who are knowledgeable about local and international regulations, industry standards, and best practices. These professionals help ensure that the CDMO stays up-to-date with the latest regulatory changes and maintains compliance throughout the life science or IVD manufacturing process.
Regular audits and inspections: CDMOs undergo regular audits and inspections by both their clients and regulatory authorities to verify that they maintain compliance with applicable regulations and quality standards. These inspections help identify potential issues and ensure that corrective actions are taken promptly.
Employee training: CDMOs invest in continuous training and education for their employees to ensure that they have the necessary skills and knowledge to maintain quality and compliance. This includes training on relevant regulations, industry standards, and best practices.
Quality control and assurance: CDMOs implement rigorous quality control (QC) and quality assurance (QA) processes throughout the life science and IVD manufacturing process. QC involves testing and monitoring of raw materials, intermediate products, and finished products to ensure that they meet established specifications. QA focuses on the overall quality management system and helps identify and address potential issues before they impact product quality.
Documentation: CDMOs maintain thorough and accurate documentation of their life science and IVD manufacturing processes, quality control results, and compliance efforts. Proper documentation is essential for demonstrating compliance with regulations and facilitating audits and inspections.
Continuous improvement: CDMOs are committed to continuous improvement in their quality and compliance efforts. They regularly review their processes, systems, and performance to identify areas for improvement and implement necessary changes.
By implementing these measures, reputable CDMOs like Argonaut can ensure that they consistently deliver high-quality products while maintaining compliance with applicable regulations. Partnering with a CDMO that prioritizes quality and regulatory compliance can provide peace of mind and help minimize potential risks associated with life science or IVD manufacturing.
A Contract Development Manufacturing Organization (CDMO), is a company that provides comprehensive services to biotech and pharmaceutical companies related to the development, manufacturing, and testing of drugs and other biotechnology products. CDMOs offer a wide range of services, including supply chain management, technical transfer, engineering development, first article builds, cGMP (current Good Manufacturing Practices) manufacturing, and quality and regulatory services.
There are several reasons why life science & diagnostic companies should consider working with a CDMO:
Expertise and experience: CDMOs possess specialized knowledge and expertise in the field of life science and IVD manufacturing. They are well-versed in the latest technologies, equipment, and regulatory requirements, ensuring that your product is manufactured to the highest quality standards.
Cost savings: By outsourcing life science and IVD manufacturing to a contract manufacturing organization, biotech companies can save on the costs of setting up, maintaining, and operating their own manufacturing facilities. This can free up resources that can be redirected towards research and development, allowing companies to focus on their core competencies.
Flexibility and scalability: CDMOs offer a high level of flexibility and can easily adapt to changing production needs. They can quickly scale up or down according to the client’s requirements, ensuring a seamless transition between different stages of the product lifecycle.
Risk mitigation: By working with a contract manufacturing organization, biotech companies can mitigate the risks associated with life science and IVD manufacturing, such as potential production delays or issues with regulatory compliance. CDMOs have established quality management systems and follow cGMP guidelines, ensuring that the final product meets regulatory requirements.
Faster time to market: CDMOs can help accelerate the product development process by taking care of manufacturing and related services, allowing biotech companies to focus on other aspects of bringing their product to market. This can result in faster time to market and a competitive advantage.
In summary Partnering with a CDMO can provide significant benefits to life science & diagnostic companies working with POCT (Point of Care Testing), IVD (Diagnostics) & RUO (Research Use Only) products, including cost savings, increased flexibility, risk mitigation, and faster time to market. By leveraging the expertise and resources of a CDMO like Argonaut, biotech companies can focus on their core competencies and successfully bring their products to market.