Foundry Model Part One – Keeping Pace in Molecular Diagnostics Innovation
The foundry model is poised to fundamentally transform the biotechnology and molecular diagnostics sectors. As it revolutionized the semiconductor industry in the mid 1980’s, the foundry model brings to molecular diagnostics companies a method to leverage the commonality of manufacturing techniques.
By joining common manufacturing processes across several companies, assay manufacturing becomes a variable cost versus a fixed cost with substantial benefits to assay developers. In fact, the underlying industry conditions that drove the foundry model in the semiconductor industry are the same as those facing molecular diagnostics today: Pace, Leverage and Cost.
In this 3-part blog series, we will take a look at each of these drivers.
First, let’s look at the Pace of innovation (In semiconductor terms, this is often referred to as Moore’s law). Even by the mid 1980’s there was a seemingly impossible pace of change and innovation in the semiconductor industry and Moore’s law was used to describe a doubling every two years in the number of components per integrated circuit. Fundamentally, this was translated into greater complexity and opportunity with each new chip.
This scenario is being replicated in Molecular Diagnostics as the advent of NextGen Sequencing plummets sequencing costs, giving rise to an enormous amount of data, and vast opportunities to better understand and control disease. Indeed, the reductions in sequencing costs (per mega-base) are outpacing Moore’s law (see graph below).
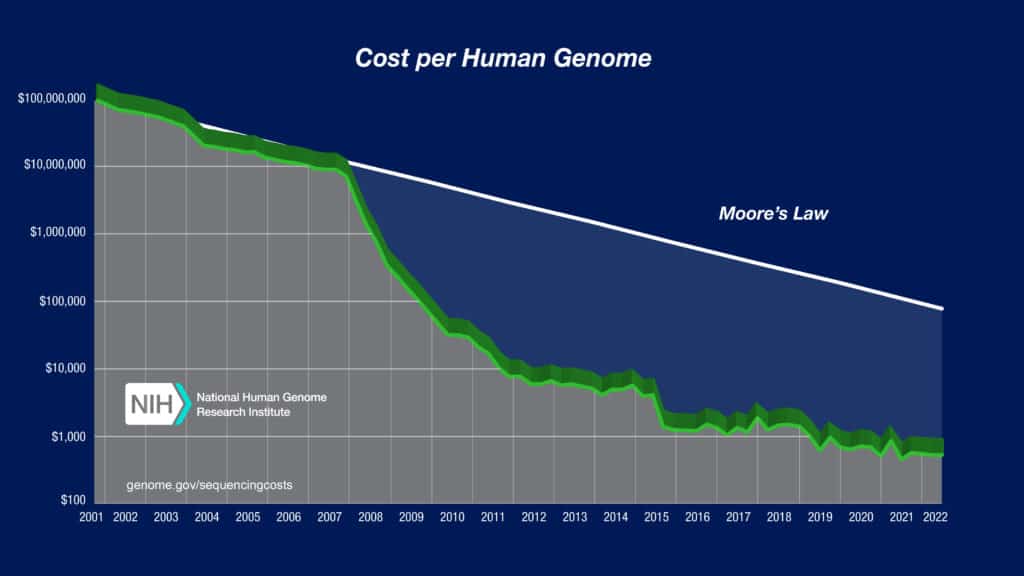
source: genome.gov/sequencingcosts
Without a solution in the Molecular Diagnostic market, the pace of innovation will likely be slowed as access to capital resources is constrained by the increasing need for R&D investment to compete in the markets. By using the Foundry Model, innovators gain access to advanced “last mile” development support, equipment, capacity and quality compliance as shared services. Previously these innovators would have struggled to afford these capabilities without significant operational investments- and these capabilities would have been highly underutilized.
Next month, in Part Two of our series, we will take a look at Leverage of Commonality in manufacturing techniques.
Want to learn more about how you can leverage the Foundry model in your organization? Contact us
You can download the full case-study: “The Foundry Model is Coming to Molecular Diagnostics, Courtesy of the Semiconductor Industry”, and find more more white papers on the Case Studies Page.
Click to read Part 2, Leveraging Commonality and Part 3, Increasing Cost of Innovation.



