With an ever increasing need to reduce our carbon footprint, we need to find alternative solutions to our shared challenges, and biotechnology- for all it’s good, must be included. The cold-chain used for shipping and storing reagents, kits, drug products, and diagnostics requires extensive electricity, fuel, and infrastructure (1) and must become greener.
“Lyophilizing assay reagents provides a solution to reduce carbon footprint on shipping and storage.”
In March 2024 ISO addressed climate change by requiring organizations that are registered to any of the ISO Management System Standards (MSS) to consider climate change within their management system. “These new inclusions are to ensure that climate change is firmly on the organization’s radar screen and is given special attention as one of the external issues to be considered in the design and implementation of their quality management system.” (2).
Argonaut has been addressing this challenge head-on since we acquired LyoGen in 2018. With LyoGen’s expertise on lyophilizing assay reagents we secured the chance to eliminate cold chain logistics and extend product shelf life of life science and diagnostic assays. As a service-oriented organization, we can assist you in reaching your objectives to contribute to a more sustainable world – while reducing total landed costs.
We can assist you in reaching your objectives to contribute to a more sustainable world – while reducing total landed costs.
Why Lyophilizing Assay Reagents Reduces Carbon Footprint
Life science reagents are fragile and traditionally need cold storage to preserve efficacy. Lyophilization quickly removes water, putting fragile reagents into stasis. Once lyophilized, cold storage is no longer required and the lyophilized assay reagents can be stored at ambient temperatures, thereby reducing the carbon footprint associated with overnight shipping, freezer farms, and temperature-controlled infrastructure. Here are the details:
Liquid reagents often need to be stored at freezer temperature, at usually between -25°C and -15°C, and sometimes as low as -80C°. Freezers need electrical energy, and storage in a -20°C freezer with a 100-kit capacity can result in creating 110kg CO2e every 90 days. Comparatively, a -80C° freezer with the same kit capacity produces 499 kg CO2e/90 days. With lyophilized reagents, cold storage CO2 emissions are completely eliminated.
| Liquid reagents | CO2e every 90 days | Liquid reagents | CO2e every 90 days | Lyophilized reagents | CO2e every 90 days |
| Storage 100 kits at -80°C | 499 kg | Storage 100 kits at -20°C | 110 kg | Storage 100 kits at room temperature | 0 kg |
Table 1: Comparing liquid reagents vs lyophilized reagents storage carbon emission
In addition to storing life science kits at cold temperatures, shipping kits to warehouses and consumers requires cold chain transportation. As an example, shipping kits with liquid reagents at required cold temperatures from Carlsbad, CA to Cambridge, MA by air results in generating more than 1,500kg CO2e. Compare this to shipping lyophilized reagents at ambient temperatures by air the same distance where it only results in 200kg CO2e – less than 1/5 of cold-shipping carbon emissions. When shipping across the globe there’s an even bigger impact. Cold-shipping by air from Los Angeles, U.S. to London, U.K. results in generation of more than 6,000kg CO2e, while shipping at ambient temperature results in 400kg CO2e – a whopping 1/15 reduction in carbon emission.
| Liquid reagents | CO2e | Lyophilized reagents | CO2e |
| Cold chain shipping CA to MA, U.S. | 1,500 kg | Ambient shipping CA to MA, USA | 200 kg |
| Cold chain shipping U.S. to U.K | 6,000 kg | Ambient shipping USA to UK | 400 kg |
Table 2: Comparing liquid reagents vs lyophilized reagents shipping carbon emission
We have covered analyzing reducing carbon emissions through ambient storage and shipping in more detail in our white paper Lyophilization and ESG (3).
Why Lyophilizing Assay Reagents Reduces Cost
In addition to reducing carbon footprint there’s an additional hidden, and easily overlooked, monetary saving to transitioning from liquid to lyophilized assay reagents. Analysis on the impact of lyophilizing assay reagents showed substantial financial savings compared to cold chain logistics when Total Landed Costs (TLC) were considered. The definition of TLC equals the sum of total (delivery-ready) purchase price of the product plus total transportation cost (including packaging and shipping).
In our white paper How to Eliminate Hidden Cold Chain Costs- Lyophilizing Diagnostic Assays (4) we compare the costs of liquid reagents under cold chain with the cost of lyophilized reagents at ambient temperature. In this white paper we use the same shipping scenarios as above. When shipping lyophilized kits from Carlsbad, CA to Cambridge, MA there’s a 16% cost savings, and as much as 43% cost savings when shipping from Los Angeles, U.S. to London, U.K. This is because kits with lyophilized reagents are both smaller and lighter than kits with liquid reagent.
| Shipping distance | % Savings using lyophilized kits |
| CA to MA, U.S. | 16% |
| U.S. to U.K. | 43% |
Table 3: Total landed cost (TLC) savings when lyophilizing assay reagents
We also need to consider that not all shipping logistics end up as planned. During the pandemic we clearly saw the fragility and complexities of global shipping logistics. A cold chain shipment will in most cases not tolerate more than a day or two of delay before the reagents are compromised. Lyophilized kits have a longer shelf life and can tolerate several days of delay and resulting in less write-offs. You can read more about this in our blog post Has COVID-19 Killed the Life Science Cold Chain Shipping?.
The definition of total landed cost (TLC) equals the sum of total purchase price of the product plus total transportation cost.
Why Lyophilizing Assay Reagents Reduces Cost – and Helps the Environment
In addition to reducing carbon footprint through ambient storage and shipping, an added benefit is the elimination of the need for polystyrene containers (exampled by Styrofoam™ boxes). This, not only saves on cost, but meets the global movement of phasing out polystyrene foam as a single use plastic (5). As of June 2024, 11 U.S. states and two territories passed statewide legislations to explicitly ban polystyrene foam. This ban is growing in the restaurant business. When this also transitions to shipping containers, the life science industry needs to find alternative solutions.
We discuss this in more detail in our blog post Protecting Your Life Science Cold Chain from Styrofoam Bans. A readily apparent solution is to eliminate the need for polystyrene containers by lyophilizing assay reagents so that kits can be shipped at ambient temperature in natural recycled materials.
Read more about cost savings and environmental impact in our white paper Increase Your Profit Margin with Lyophilization: Benefits for Reagent Kits & Diagnostics (6).
Lyophilization Formats and Solutions
Commonly we think of lyophilizing assay reagents as a powder formulation. While Argonaut can make lyophilized powders, there are also different formats available that can increase an assay or kit precision, while simplifying ease of use.
– Benefits of Lyophilized Beads Versus Powder
Argonaut has developed a unique process for making lyophilized beads. The beads contain a specific volume of reagents and can easily be transferred to tubes or plates. Argonaut’s highly uniform LyoDose™ beads can range from 5µl to 60µl per bead.

Bead formats have significant advantages over powder as each bead is a unit dose. This means that a single bead can be configured to deliver a specific dose of reagent. An example is a bead configured to deliver PCR MasterMix for a 20µl reaction or the bead can be reconfigured to deliver PCR MasterMix for a 40µl reaction. Beads can easily be pre-aliquoted and two or more sets of reagents in separate beads can be stored together in the same tube, where the reagents are not mixed before they are rehydrated by the end-user. In addition to lyophilizing assay reagents into beads, Argonaut can add an inert dye to each unique set of beads allowing the end-user to easily identify each bead.
Read more about beads versus powder in our popular blog post 3 Reasons Why Lyophilized Beads are the Next Big Thing in Assay Design.
– LyoDots® – The World’s Smallest Lyophilized Reagents
The LyoDose beads described above are suitable for tube or plate (96 or 384 wells) formats. Based on the LyoDose bead technology, Argonaut developed a new format called LyoDots™ designed for cartridges or automation. LyoDots allows for lyophilizing assay reagents from less than 5µl. Similar to LyoDose beads LyoDots can have different inert dyes added, and can in also be customized for various sizes specific to your product.
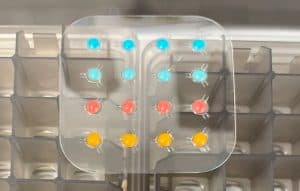
The LyoDots format is particularly suitable for point of care testing (POCT) devices. The format allows for easy integration into device assembly, accommodating small, shallow depth spaces. In addition, the shape allows for the lyophilized material to be readily dissolved. You can learn more about LyoDots in our white paper LyoDots: A Unique Lyophilization Format for Next Generation Point of Care Testing (7).
– Customized Lyophilized Formats
One size does not fit all, and for several projects we have accommodated various lyophilization formats. We can do lyophilization in-situ in various formats in vials, tubes, strips as well as custom solutions. Get in touch with us and we will find a format that fits your project.
– BioFuse™ Sample Prep – Simplify your Molecular Assay
Argonaut has developed a solution where pipetting can be eliminated in assays, both improving end-user experience while also reducing user errors. Customized LyoDose beads are placed into a BioFuse Sample Prep device where they are stored at room temperature until used. Users simply add a test sample to the BioFuse tube, twist on the BioFuse Sample Prep cap, rotate the tube to dissolve the beads with the sample, snap the tip off the cap and squeeze a calibrated drop into the testing device. No-pipetting needed. See a video of how BioFuse Sample Prep works here.

Lyophilizing Assay Reagents to Your Needs
Earlier we described how organizations can save on cost and positively impact the environment by utilizing lyophilization technologies. There are two common concerns: 1) the assay or kit reagents can’t be lyophilized (e.g. components contain high glycerol concentrations) and 2) uncertainty over lyophilizing reagents in-house, or whether it is better to outsource.
– How to Lyophilize Your Assay Reagents
It is correct that not all reagents and formats can be lyophilized, and that lyophilizing assay reagents can be tricky, but Argonaut has experts that can perform the evaluation. A thermal analysis is performed to ensure the best chance of successfully lyophilizing the formulation you provide us. We help you optimize your formulation by doing a feasibility study where we test various conditions to your reagent mixture. Argonaut uses a range of select excipients to facilitate the lyophilization process, and we test these excipients across multiple lyophilization settings. We want to make sure you get what you need to your formulation specifications. You can read our Comprehensive Guide to Outsourcing Reagent & Assay Lyophilization and How to Overcome Your Protein Lyophilization Roadblocks to learn more.
Argonaut has experts that can evaluate if your formulation can be lyophilized.
– Outsource or Do-it-Yourself
There are many different processes that can be used for lyophilizing assay reagents. But beyond the technical choices, teams need to consider whether to pursue an in-house or outsourced lyophilization solution. Some questions to consider include: Does your organization have the time and resources to find the best procedure for lyophilizing reagents? Will the organization have the required low humidity facilities and capital equipment? Importantly, does your team have resources to train your staff or hire people with the right skillset? We discuss this and more in the blog post Lyophilization Process- The Argument for Specialization.
At Argonaut we have the ability to get your assay lyophilized:
- We are the lyophilization experts.
- We have a state-of-the-art lyophilization facility with numerous lyophilization technologies.
- Our staff has been lyophilizing assay reagents for decades.
Does your team have resources to train your staff or hire people with the right lyophilization skillset?
Argonaut provides a full service, turn-key framework that builds your assay from raw material through formulation, lyophilization, kitting, labeling, and packaging – as well as storage and shipping. Our lyophilization platforms incorporate a comprehensive network of ecosystem partners ensuring streamlined solutions, lyophilization ready reagents, and reliable results. Importantly, all our services are cGMP and ISO 13485:2016, as Argonaut works under multiple other licenses, certifications and registrations.
You can read more about our turn-key manufacturing services and why you can benefit from choosing Argonaut as your contract manufacturing partner here.
Contact us to discuss how we can help you with lyophilizing your assay reagents.
References
(1) Grand Challenges in Pharmaceutical Research Series: Ridding the Cold Chain for Biologics https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7869771/
(2) ISO 9001 Auditing Practices Group Guidance on: Auditing Climate Changes issues in ISO 9001 https://committee.iso.org/files/live/sites/tc176/files/PDF%20APG%20New%20Disclaimer%2012-2023/APG%20Auditing%20Climate%20Change%20issues%20FINAL%203-19-2024%20Rev%201.pdf
(3) Lyophilization and ESG. Reducing Carbon Emission (CO2e) of In-Vitro Diagnostic (IVD) Assays and Reagents Using Lyophilization https://www.argonautms.com/wp-content/uploads/2023/09/Lyophilization-and-ESG-Reducing-Carbon-Footprint-of-Assays.pdf
(4) How to Eliminate Hidden Cold Chain Costs- Lyophilizing Diagnostic Assays. The Ture Costs of Cold Chain Logistics Are Not What You Think https://www.argonautms.com/wp-content/uploads/2023/04/Lyophilizing-Diagnostic-Assays-TLCold-Chain-Apr-2023.pdf
(5) Phase-out of polystyrene foam https://en.wikipedia.org/wiki/Phase-out_of_polystyrene_foam
(6) Increase Your Profit Margin with Lyophilization: Benefits for Reagent Kits & Diagnostics https://www.argonautms.com/wp-content/uploads/2022/01/Argonaut-Benefits-of-Lyophilization.pdf
(7) LyoDots™ (lyophilized reagents in a flat, circular shape): A Unique Lyophilization Format for Next Generation Point of Care Testing https://www.argonautms.com/wp-content/uploads/2022/07/LyoDot-White-Paper-2022-07-21.pdf



